 |
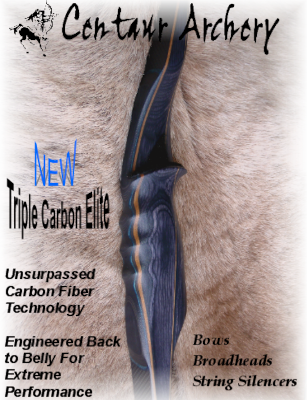   < < |
 |

|

|
| INFO: Trad Archery for Bowhunters |

- Welcome to Trad Gang.
Not another Quench Oil Post!!
Started by kbaknife, December 18, 2009, 09:41:00 AM
Previous topic - Next topic0 Members and 1 Guest are viewing this topic.
User actions
Copyright 2003 thru 2025 ~ Trad Gang.com © |
